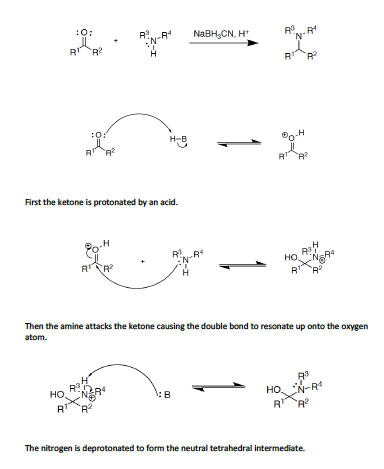


The Borch Reductive Amination reaction is one which is quite common and used in order to produce alkylated amines. A carbonyl group is used in the reaction and is converted to an amine through an imine intermediate. Sodium Cyanoborohydride (NaBH3CN) is commonly used in these reactions because it is a weak reducing agent which acts under mildly acidic conditions. It is important to monitor the pH of this reaction in order for it to go to completion. When reacting with ketones and aldehydes the reaction should take place in a pH of 3-5. When reacting with iminium cations a pH of 6-7 should be used. Here is an animation of the reaction as well as a tutorial of the reaction with explanations after each step. The R1, R2, R3, and R4 groups all represent different carbon groups connected to the carbonyl and amine atoms.

.png)
